How many electrons are in the ion p 3 a 12 b 34 c 18. How many total electrons does the P3- ion have.

How To Find Protons Electrons For The Phosphide Ion P 3 Youtube
The given ion contains 37 protons in.

. In addition there are hundreds of compounds in which phosphorus is present as an anion a negatively charged ion. See answer 1 P3- ion has 15 protons as the atomic number of phosphorus is 15 and 18 electrons 15 electrons from phosphorus and three from the negative charge. How many electrons are in the ion Cu2.
Pages 4 This preview shows page 1 - 3 out of 4 pages. So P3- has 18 electrons. The number of electrons is 18 The number of electrons is 18 Question 7 Difficulty.
What is the total number of electrons in a p3- ion. Answer 1 of 10. A 27 B 29 C 31 D 64.
Give the number of protons in Na1. 31 P 3- protons Z 15 neutrons A - Z 31 - 15 16 Charge is 3- so there are 3 more electrons than protons and so the number of electrons 18. A pure phosphorus cation.
Likewise how many neutrons does p3 have. How many outer electrons does have FAQconsider the electron configuration for iron. An ion has 8 protons 9 neutrons and 10 electrons.
In which of the following sets do all species have the same number of. Phosphorus has 15 electrons. A 18 B 12 C 19 D 15 E 16.
A charge of -3 indicates that it has gained 3 electrons. Hope this helps. The symbol for the ion is ________.
Therefore the ion contains 36 electrons. P3- is formed when three electrons are added to phosphorus and hence it has 18 electrons. P3- has 18 electrons.
How many electrons are in the ion P 3 a 12 b 34 c 18 d 28 6 Electrons located in. The number of protons will remain same that is the number of protons 15. The atomic number of an atom is the number of protons in its nucleus.
Isotopes differ in the number of. P3- Phosphide ion is a negative ionanion formed when the non-metal atom phosphorusP gains 3 extra electrons in its outermostvalence shell. Science Chemistry QA Library How many protons p neutrons n and electrons e does the following ion have.
That means it has in its neutral state. Similarly is Phosphorus a positive or negative ion. Therefore it has 15 protons.
Course Title CHEM 1030. The electron configuration of _15P is. Oxygen and Nitrogen doesnt have -1 valency.
Similarly one may ask what is the electron configuration of p3 -. 1s22s22p63s23p6 The electron configuration of _42Mo is. In its valence shell P has only 5 electrons.
The main known forms of the element are white red and black phosphorus. Solutions for Chapter 4 Problem 73QP. A 10 B 13 C 9 D 11 E 12.
8 electrons and it must be Flourine. Phosphorus has atomic number 15 it means that it has 15 protons as well as 15 electrons because atom is neutral. P Phosphorus has an atomic number of 15.
How many electrons are in the ion P3-. Therefore it has 18 electrons. 0 1 0 What type of bonding is found in the compound NH 3.
Also how many electrons are in the ion p3 -. The atomic number of P is 15. Give the number of electrons in P-3.
But here we have P3- which means that this atom has gained 3 electrons. 1s22s22p63s23p3 When phosphorous gains 3 electrons to form the ion P3- the electron configuration becomes. Hereof how many electrons are in the ion p3 -.
The symbol is for phosphorus having atomic number 15. P 3-1s 2 2s 2 2p 6 3s 2 3p 6. Get solutions Get solutions Get solutions done loading Looking for the textbook.
The atomic number of Flourine is 9 so it will recieve a single electron to become F- ion with -1 valency. How many outer electrons does have adminSend emailDecember 10 2021 minutes read You are watching consider the electron configuration for iron. 31 O c1 O d.
P3- Group of answer choices 31p. The given symbol has a -3 charge that means it has gained 3 electrons so the number of electrons in the is. A 12 B 18 C 28 D 34.
How many electrons are present in each of the following ionsa. An atom that has gained an electron.

How To Find Protons Electrons For The Phosphide Ion P 3 Youtube
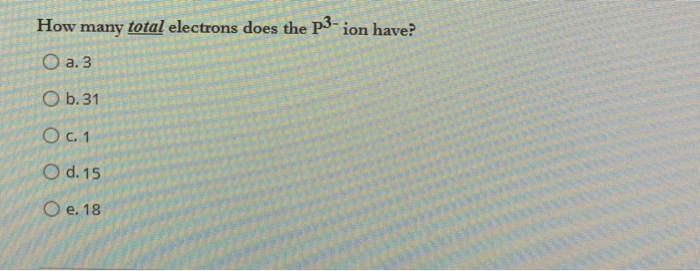
Solved How Many Total Electrons Does The P3 Ion Have O A Chegg Com
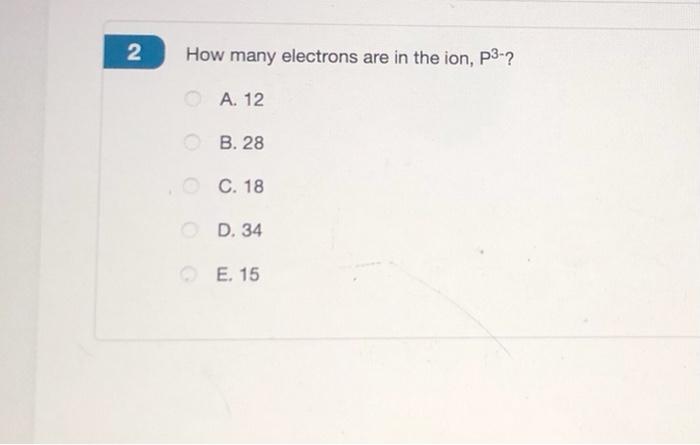
Solved 2 How Many Electrons Are In The Ion P3 A 12 B 28 Chegg Com
0 Comments